1 Flame retardant system selection
This paper mainly studies ultra-thin fire-retardant coatings applied to steel structures, and the coating thickness should be controlled below 2mm. At present, the fire protection system in the field of fire retardant fireproof coating mainly includes two types of expansion type and non-expansion type. Non-intumescent fireproof coatings are mainly filled with flame retardant flame retardants, binders and fireproof fillers because they do not have an expansion effect. They do not expand substantially during the fire baking process, but rely solely on the non-flammability of the coating itself. Low heat transfer and heat absorption, retarding the transfer of heat and delaying the temperature rise of steel. If the effect of fire and heat insulation is to be achieved, it can only be achieved by increasing the thickness of the coating. It is not an ultra-thin fireproof coating, so it is not within the scope of this paper.
In this study, the typical outdoor weathering powder coating was used as the main body, and the flame retardant effect of the coating under different flame retardant systems was compared. The results are shown in Table 1.
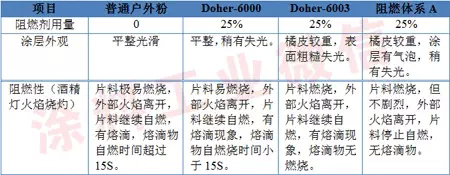
Table 1 Effect of flame retardant system
The test results show that the conventional powder coating does not have the effect of flame retardant and fireproof, and the ordinary intumescent halogen-free flame retardant Doher-6000 can play a certain flame retardant effect, but the effect is not obvious; the flame retardant Doher-6003 belongs to the nitrogen system. Halogen-free flame retardant, its fireproof effect is better than Doher-6000, but there are still droplets; flame retardant system A belongs to composite expansion flame retardant system, from acid source (ammonium polyphosphate, type I), gas source ( Melamine is combined with a carbon source (pentaerythritol), and its flame retardant effect is the best among the above three flame retardant systems. When the alcohol lamp is burned, no droplets are generated. Once the alcohol lamp flame leaves, the sheet stops. Combustion indicates that the flame retardant effect is very good, so A is selected as the flame retardant system for the test in the subsequent tests. In addition, the addition of flame retardant has an adverse effect on the appearance of the coating. In the above 3 flame retardant systems, the influence of Doher-6000 and Doher-6003 on the appearance of the coating is relatively small, while the intumescent flame retardant system A is coated. The effect of the appearance is much greater, which is related to the decomposition of the flame retardant in the heat-cured baking process, which will be studied in subsequent tests.
2 Research on flame retardant system
2.1 Research on the main body of flame retardant
Flame Retardant System A is a composite flame retardant, which consists of the following three parts:
(1) Acid source: its role is to decompose in the event of fire, produce inorganic acid or organic acid, dehydration and carbonization of polyol, from the source and cost of raw materials, the price of ammonium polyphosphate (APP, type I) is low, It has low toxicity, good dispersibility and wide range of sources. Therefore, this study uses it as the acid source of the flame retardant system.
(2) Charcoal source: Its main function is to react and decarbonize with a dehydration carbon catalyst (acid source) in the event of fire. The formed carbonization layer protects the steel from heat transfer and forms carbon in the intumescent fireproof coating. The decomposition temperature of the agent should be matched with the dehydration catalyst. Therefore, when ammonium polyphosphate is used as the catalyst for dehydration to carbon, the high carbon polyhydroxy compound with high thermal stability is generally used as a carbon-forming agent for the flame retardant system, from cost, source and In view of the carbonization effect, pentaerythritol (PE) is suitable as a carbonization agent for an intumescent fireproof coating.
(3) Gas source: Its main function is to decompose and release gas in the event of fire to expand the carbonized layer, because the expanded carbonized layer has more obvious heat insulation effect. At present, domestic intumescent fireproof coatings generally use melamine (MEL) as a foaming agent. Because the thermal decomposition temperature of MEL is similar to the thermal decomposition temperature between APP and PE, the three can exert better synergistic effects.
The thermal decomposition behavior of the above three substances was determined in the study process (see Figure 1 for the results).
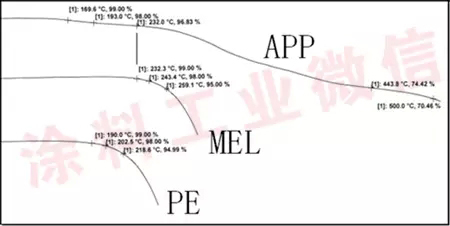
Figure 1 Thermal decomposition curve of each component of flame retardant
The results showed that ammonium polyphosphate (acid source, APP, type I) began to decompose slightly at around 170 °C, and its decomposition process was relatively smooth, while melamine (gas source, MEL) began to decompose rapidly from 230 ° C, pentaerythritol (carbon source, PE) starts to decompose rapidly from 190 °C. Since the ambient temperature during the actual fire occurrence is obviously higher than 170 ° C (actually should be between 800 and 1000 ° C), the acid source first decomposes during the fire, which in turn causes carbonization of the carbon source to form a carbonized layer. As the temperature rises, the gas source begins to decompose, releasing NH3, NO2 and other gases, causing the carbonized layer to expand, and finally forming a protection against the steel structure by heat insulation.
The above analysis shows that the flame retardant system selected in this project is consistent with the original idea of ​​the test. At the same time, it is easy to see from the thermal decomposition curve that the decomposition reaction occurs at 200 ° C, especially ammonium polyphosphate (type I), and the decomposition reaction occurs initially at 170 ° C, and the decomposition reaction is as a formula. 1 is shown.
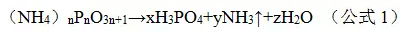
The typical curing temperature range of powder coatings is generally between 180 and 200 ° C. When curing in this temperature range, the flame retardant partially decomposes and releases small molecules (NH3 and H2O), thereby affecting the appearance of the coating. The effect of fire retardant coating after curing. To solve the problem of decomposition of the flame retardant before curing of the coating film, it can be achieved by lowering the curing temperature of the coating to below 170 ° C or increasing the decomposition temperature of the flame retardant (that is, using ammonium polyphosphate having a relatively high decomposition temperature).
This study first compared the differences in the thermal decomposition of two different types of APP (type I, type II) in the same type of powder coating system. The results are shown in Figure 2. The test results show that the initial decomposition temperature of type II APP is nearly 10 °C higher than that of type I APP in the same formulation system. Therefore, in the future test, type II APP was used as the acid source. After using Type II APP, the bubbles of the film after curing are significantly reduced, and the appearance is improved.
Figure 2 Thermal decomposition curve of fire retardant coating
In the research process of this paper, a series of tests were carried out to compare the expansion of the coating under different flame retardant distribution ratios after exposure to open flame. The results are shown in Table 2.
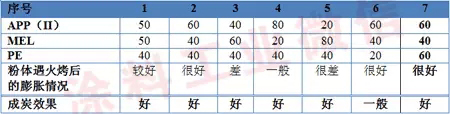
Table 2 Effect of distribution ratio of flame retardant system on coating expansion effect
The experimental results show that the composition ratio of acid source (APP) and gas source (MEL) can achieve better expansion effect at 3/2. At this ratio, N atom and P atom can achieve better compounding effect. Excessive or insufficient ratio will affect the expansion of the coating after fire. The carbon source (pentaerythritol, PE) does not contain N/P atoms, and its role is mainly to form charcoal during combustion. Therefore, the carbon content of the coating after combustion can be controlled by adjusting the content of PE. The test data indicates that the PE content can reach 40g. Achieving a good charcoal effect, continuing to increase the amount, the char forming effect does not change significantly, so it is more suitable to add about 40 g of a carbon-forming agent per 200 g of resin (including polyester resin and epoxy resin) in the formulation. Therefore, the flame retardant system in this study was determined as: APP/MEL=3/2, and the amount of PE added was 40g/200g resin. The formula will be designed according to this standard in future tests.
2.2 Other flame retardant additives
In the actual fire occurrence process, when the fireproof coating is burned by the flame, a large amount of smoke will be released. Therefore, some smoke suppressant is generally added to the fireproof coating, and the amount of the fireproofing agent is generally 2 to 4% of the total amount of the powder coating. In this study, zinc oxide (ZnO) was selected as a smoke suppressant in the formulation.
Precession Vortex Flowmeter,Precession Vortex Flow Meter,Precession Vortex Gas Flow Meter,Hydraulic Flow Meter
Wuxi Winsun Automation Instrument Co., Ltd , https://www.jswxwinsun.com